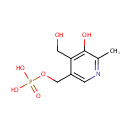
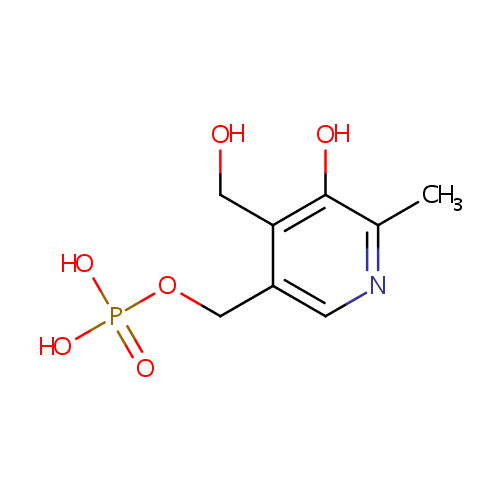
Pyridoxine 5'-phosphate (PAMDB000338)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000338 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Pyridoxine 5'-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Pyridoxine 5'-phosphate is a substrate for Pyridoxine-5'-phosphate oxidase and Pyridoxal kinase. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C8H12NO6P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 249.1577 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 249.040223633 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | WHOMFKWHIQZTHY-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C8H12NO6P/c1-5-8(11)7(3-10)6(2-9-5)4-15-16(12,13)14/h2,10-11H,3-4H2,1H3,(H2,12,13,14) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 447-05-2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[5-hydroxy-4-(hydroxymethyl)-6-methylpyridin-3-yl]methoxy}phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | pyridoxine-5'-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC1=NC=C(COP(O)(O)=O)C(CO)=C1O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as pyridoxine-5'-phosphates. These are pyridoxines that carry a phosphate group at the 5-position. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pyridines and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyridoxines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyridoxine-5'-phosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 1-Deoxy-D-xylulose 5-phosphate + NAD + O-Phospho-4-hydroxy-L-threonine > Carbon dioxide + Hydrogen ion +2 Water + NADH + Pyridoxine 5'-phosphate + Phosphate Water + Pyridoxine 5'-phosphate > Phosphate + Pyridoxine Adenosine triphosphate + Pyridoxine <> ADP + Pyridoxine 5'-phosphate 3-Amino-2-oxopropyl phosphate + 1-Deoxy-D-xylulose 5-phosphate + 3-Amino-2-oxopropyl phosphate <> Pyridoxine 5'-phosphate + Phosphate +2 Water 1-Amino-propan-2-one-3-phosphate + 1-Deoxy-D-xylulose 5-phosphate > Hydrogen ion + Pyridoxine 5'-phosphate + Phosphate + Water Adenosine triphosphate + Pyridoxine > Hydrogen ion + ADP + Pyridoxine 5'-phosphate Oxygen + Pyridoxine 5'-phosphate > Hydrogen peroxide + Pyridoxal 5'-phosphate Pyridoxamine 5'-phosphate + Water + Oxygen + Pyridoxine 5'-phosphate <> Pyridoxal 5'-phosphate + Ammonia + Hydrogen peroxide 1-Deoxy-D-xylulose 5-phosphate + 2-Amino-3-phosphonopropionic acid + 1-Deoxy-D-xylulose 5-phosphate + 2-Amino-3-phosphonopropionic acid > Pyridoxine 5'-phosphate + Phosphate + Hydrogen ion +2 Water Pyridoxine + Adenosine triphosphate > Pyridoxine 5'-phosphate + Adenosine diphosphate + Hydrogen ion + ADP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Argoudelis, Chris J. Preparation of crystalline pyridoxine 5'-phosphate and some of its properties. Journal of Agricultural and Food Chemistry (1986), 34(6), 995-8. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||