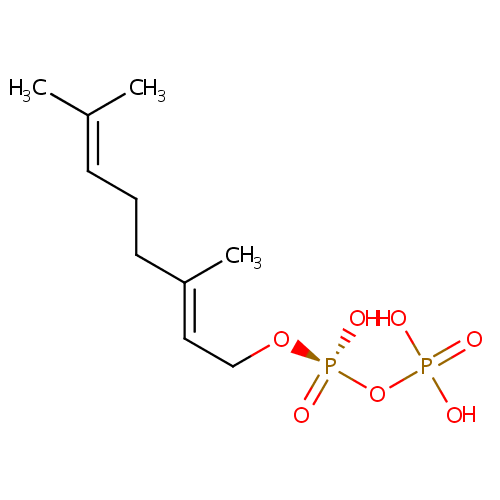
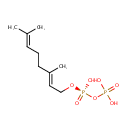Geranyl-PP (PAMDB000324)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000324 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Geranyl-PP | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Geranyl diphosphate is regarded as a key intermediate in the steroid, isoprene and terpene biosynthesis pathways and is used by organisms in the biosynthesis of farnesyl pyrophosphate, geranylgeranyl pyrophosphate, cholesterol, terpenes and terpenoids. (wikipedia). In E.coli, geranyl diphosphate synthase (GPPS) catalyzes the condensation of dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP) to form geranyl diphosphate. Geranyl pyrophosphate is an intermediate in the HMG-CoA reductase pathway used by organisms in the biosynthesis of terpenes and terpenoids. (Wikipedia) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C10H20O7P2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 314.2091 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 314.068426018 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | GVVPGTZRZFNKDS-JXMROGBWSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C10H20O7P2/c1-9(2)5-4-6-10(3)7-8-16-19(14,15)17-18(11,12)13/h5,7H,4,6,8H2,1-3H3,(H,14,15)(H2,11,12,13)/b10-7+ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 763-10-0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | [({[(2E)-3,7-dimethylocta-2,6-dien-1-yl]oxy}(hydroxy)phosphoryl)oxy]phosphonic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | geranyl diphosphate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(C)=CCC\C(C)=C\CO[P@@](=O)(O)OP(=O)(O)O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as isoprenoid phosphates. These are prenol lipids containing a phosphate group linked to an isoprene (2-methylbuta-1,3-diene) unit. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Prenol lipids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Isoprenoid phosphates | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Isoprenoid phosphates | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Dimethylallylpyrophosphate + Isopentenyl pyrophosphate > Geranyl-PP + Pyrophosphate Geranyl-PP + Isopentenyl pyrophosphate + Geranyl diphosphate <> Farnesyl pyrophosphate + Pyrophosphate Geranyl-PP + Isopentenyl pyrophosphate <> Pyrophosphate + Farnesyl pyrophosphate all-trans-Polyprenyl diphosphate + 4-Hydroxybenzoic acid + Geranyl-PP <> 4-Hydroxy-3-polyprenylbenzoate + Pyrophosphate + 4-Hydroxy-3-polyprenylbenzoate Geranyl-PP + 1,4-Dihydroxy-2-naphthoic acid <> Demethylmenaquinol + Pyrophosphate + Carbon dioxide Dimethylallylpyrophosphate + Isopentenyl pyrophosphate + Dimethylallylpyrophosphate + Isopentenyl pyrophosphate > Pyrophosphate + Geranyl-PP + Geranyl-PP Geranyl-PP + Isopentenyl pyrophosphate + Geranyl-PP + Isopentenyl pyrophosphate > Pyrophosphate + Farnesyl pyrophosphate + Farnesyl pyrophosphate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Runquist M; Ericsson J; Thelin A; Chojnacki T; Dallner G Biosynthesis of trans,trans,trans-geranylgeranyl diphosphate by the cytosolic fraction from rat tissues. Biochemical and biophysical research communications (1992), 186(1), 157-65. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||