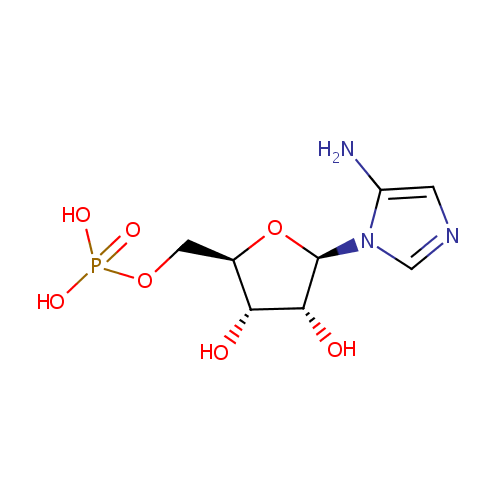
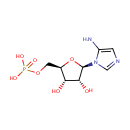5-Aminoimidazole ribonucleotide (PAMDB000306)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000306 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 5-Aminoimidazole ribonucleotide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 5-aminoimidazole ribonucleotide (AIR), is an intermediate of purine nucleotide biosynthesis, the precursor to 4-amino-2-methyl-5-hydroxymethylpyrimidine (HMP), the first product of the pyrimidine biosynthesis in a reaction mediated by the enzyme HMP-P kinase (ThiD). HMP is a precursor of thiamin phosphate (TMP), and subsequently to thiamin pyrophosphate (TPP), an essential cofactor in all living systems that plays a central role in metabolism. 5-Aminoimidazole ribonucleotide is a substrate for Scaffold attachment factor B2, Multifunctional protein ADE2, Serine/threonine-protein kinase Chk1, Vinexin, Trifunctional purine biosynthetic protein adenosine-3, Antileukoproteinase 1 and Scaffold attachment factor B. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C8H14N3O7P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 295.1864 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 295.056936329 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | PDACUKOKVHBVHJ-XVFCMESISA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C8H14N3O7P/c9-5-1-10-3-11(5)8-7(13)6(12)4(18-8)2-17-19(14,15)16/h1,3-4,6-8,12-13H,2,9H2,(H2,14,15,16)/t4-,6-,7-,8-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 25635-88-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(2R,3S,4R,5R)-5-(5-amino-1H-imidazol-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | 5-aminoimidazole ribotide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | NC1=CN=CN1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as 1-phosphoribosyl-imidazoles. These are organic compounds containing the imidazole ring linked to a ribose phosphate through a 1-2 bond. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Imidazole ribonucleosides and ribonucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | 1-phosphoribosyl-imidazoles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | 1-phosphoribosyl-imidazoles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 5-Aminoimidazole ribonucleotide + Adenosine triphosphate + Hydrogen carbonate > 5-Phosphoribosyl-5-carboxyaminoimidazole + ADP + Hydrogen ion + Phosphate Adenosine triphosphate + Phosphoribosylformylglycineamidine <> ADP + 5-Aminoimidazole ribonucleotide +2 Hydrogen ion + Phosphate 5-Aminoimidazole ribonucleotide + Water + NAD > 4-Amino-2-methyl-5-phosphomethylpyrimidine +2 Formic acid +3 Hydrogen ion + NADH 4-Amino-5-hydroxymethyl-2-methylpyrimidine + S-Adenosylmethionine <> 5-Aminoimidazole ribonucleotide + 4-Amino-2-methyl-5-phosphomethylpyrimidine + 5'-Deoxyadenosine + L-Methionine + Formic acid + CO Adenosine triphosphate + Phosphoribosylformylglycineamidine <> ADP + Phosphate + 5-Aminoimidazole ribonucleotide Adenosine triphosphate + 5-Aminoimidazole ribonucleotide + Hydrogen carbonate <> ADP + Phosphate + 5-Carboxyamino-1-(5-phospho-D-ribosyl)imidazole + 5-carboxyamino-1-(5-phospho-D-ribosyl)imidazole 5-Aminoimidazole ribonucleotide + S-Adenosylmethionine 4-Amino-2-methyl-5-phosphomethylpyrimidine + 5'-Deoxyadenosine + L-Methionine + Formic acid + carbon monoxide + Hydrogen ion 5-Aminoimidazole ribonucleotide + Adenosine triphosphate + Hydrogen carbonate > Hydrogen ion + N5-Carboxyaminoimidazole ribonucleotide + ADP + Phosphate Adenosine triphosphate + 2-(Formamido)-N(1)-(5-phospho-D-ribosyl)acetamidine > ADP + Inorganic phosphate + 5-Aminoimidazole ribonucleotide Adenosine triphosphate + 5-Aminoimidazole ribonucleotide + Carbonic acid > ADP + Inorganic phosphate + 5-carboxyamino-1-(5-phospho-D-ribosyl)imidazole 5-Amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxylate <> 5-Aminoimidazole ribonucleotide + Carbon dioxide 2-(Formamido)-N1-(5-phospho-D-ribosyl)acetamidine + Adenosine triphosphate > 5-Aminoimidazole ribonucleotide + Phosphate + Adenosine diphosphate + Hydrogen ion + ADP 5-Aminoimidazole ribonucleotide + Hydrogen carbonate + Adenosine triphosphate > N5-Carboxyaminoimidazole ribonucleotide + Adenosine diphosphate + Phosphate +2 Hydrogen ion + N5-Carboxyaminoimidazole ribonucleotide + ADP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Groziak M P; Bhat B; Leonard N J Nonenzymatic synthesis of 5-aminoimidazole ribonucleoside and recognition of its facile rearrangement. Proceedings of the National Academy of Sciences of the United States of America (1988), 85(19), 7174-6. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||