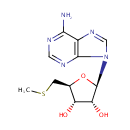
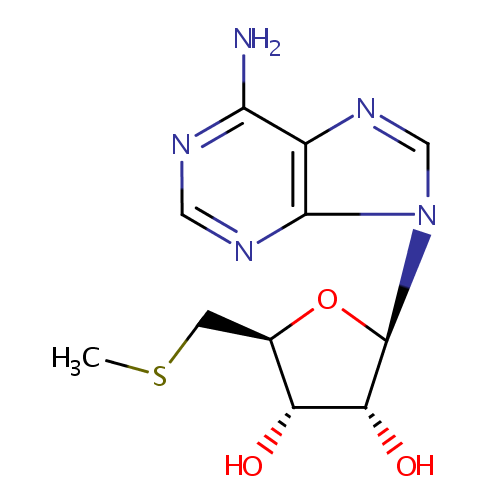
5'-Methylthioadenosine (PAMDB000282)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000282 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 5'-Methylthioadenosine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 5-Methylthioadenosine is a normal metabolite in Cysteine and methionine metabolism. Spermidine synthase (putrescine aminopropyltransferase)(EC:2.5.1.16) catalyzes the its formation and it is then converted to 5-Methylthio-D-ribose via S-adenosylhomocysteine/5'-methylthioadenosine nucleosidase [EC:3.2.2.9]. (KEGG) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C11H15N5O3S | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 297.334 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 297.089560061 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | WUUGFSXJNOTRMR-IOSLPCCCSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C11H15N5O3S/c1-20-2-5-7(17)8(18)11(19-5)16-4-15-6-9(12)13-3-14-10(6)16/h3-5,7-8,11,17-18H,2H2,1H3,(H2,12,13,14)/t5-,7-,8-,11-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 2457-80-9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methylsulfanyl)methyl]oxolane-3,4-diol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | methylthioadenosine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=NC2=C1N=CN=C2N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as glycosylamines. These are compounds consisting of an?amine?with a?beta-N-glycosidic bond?to a carbohydrate, thus forming a cyclic?hemiaminal ether?bond (alpha-amino ether). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organooxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carbohydrates and carbohydrate conjugates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Glycosyl compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Glycosylamines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | S-Adenosylmethioninamine + Putrescine + Ethylenediamine <> 5'-Methylthioadenosine + Hydrogen ion + Spermidine Cadaverine + S-Adenosylmethioninamine > 5'-Methylthioadenosine + Hydrogen ion + Aminopropylcadaverine 5'-Methylthioadenosine + Water > 5-Methylthioribose + Adenine S-Adenosylmethioninamine + Putrescine <> 5'-Methylthioadenosine + Spermidine S-Adenosylmethioninamine + Spermidine <> 5'-Methylthioadenosine + Spermine S-Adenosylmethioninamine + Cadaverine <> 5'-Methylthioadenosine + Aminopropylcadaverine Putrescine + S-Adenosylmethioninamine > Hydrogen ion + Spermidine + 5'-Methylthioadenosine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Sufrin, Janice R.; Spiess, Arthur J.; Kramer, Debora L.; Libby, Paul R.; Porter, Carl W. Synthesis and antiproliferative effects of novel 5'-fluorinated analogs of 5'-deoxy-5'-(methylthio)adenosine. Journal of Medicinal Chemistry (1989), 32(5), 997-1001. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||