FMNH (PAMDB000273)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000273 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | FMNH | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | FMNH is a member of the chemical class known as Flavins. These are compounds containing a flavin (7,8-dimethyl-benzo[g]pteridine-2,4-dione) moiety, whose structure is characterized by an isoalloaxzine tricyclic ring. During their use in different catalytic cycles, the reversible interconversion of oxidized (FMN), semiquinone (FMNH | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
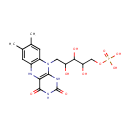
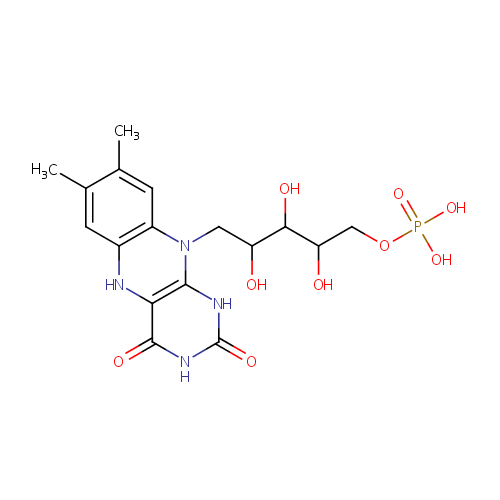
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C17H23N4O9P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 458.3597 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 458.120264866 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | YTNIXZGTHTVJBW-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C17H23N4O9P/c1-7-3-9-10(4-8(7)2)21(15-13(18-9)16(25)20-17(26)19-15)5-11(22)14(24)12(23)6-30-31(27,28)29/h3-4,11-12,14,18,22-24H,5-6H2,1-2H3,(H2,27,28,29)(H2,19,20,25,26) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | [(5-{7,8-dimethyl-2,4-dioxo-1H,2H,3H,4H,5H,10H-benzo[g]pteridin-10-yl}-2,3,4-trihydroxypentyl)oxy]phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | (5-{7,8-dimethyl-2,4-dioxo-1H,3H,5H-benzo[g]pteridin-10-yl}-2,3,4-trihydroxypentyl)oxyphosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC1=CC2=C(C=C1C)N(CC(O)C(O)C(O)COP(O)(O)=O)C1=C(N2)C(=O)NC(=O)N1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as alkyldiarylamines. These are tertiary alkylarylamines having two aryl and one alkyl groups attached to the amino group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organonitrogen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Amines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Tertiary amines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Alkyldiarylamines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Flavin Mononucleotide + Hydrogen ion + NADH > FMNH + NAD Flavin Mononucleotide + Hydrogen ion + NADPH <> FMNH + NADP FMNH + Oxygen + Sulfoacetate > Flavin Mononucleotide + Glyoxylic acid + Hydrogen ion + Water + Sulfite FMNH + Isethionic acid + Oxygen > Flavin Mononucleotide + Glycolaldehyde + Hydrogen ion + Water + Sulfite FMNH + Methanesulfonate + Oxygen > Formaldehyde + Flavin Mononucleotide + Hydrogen ion + Water + Sulfite Butanesulfonate + FMNH + Oxygen > Butanal + Flavin Mononucleotide + Hydrogen ion + Water + Sulfite Ethanesulfonate + FMNH + Oxygen > Acetaldehyde + Flavin Mononucleotide + Hydrogen ion + Water + Sulfite 2 Ferroxamine + FMNH >2 Iron +2 ferroxamine minus Fe(3) + Flavin Mononucleotide +2 Hydrogen ion Uracil + FMNH + Oxygen <> Ureidoacrylate peracid + Flavin Mononucleotide NAD(P)<sup>+</sup> + FMNH <> NAD(P)H + Flavin Mononucleotide + Hydrogen ion Thymine + Oxygen + FMNH > (<i>Z</i>)-2-methylureidoacrylate peracid + Flavin Mononucleotide + Hydrogen ion an alkanesulfonate + Oxygen + FMNH > an aldehyde + Sulfite + Water + Flavin Mononucleotide + Hydrogen ion Uracil + Oxygen + FMNH > Hydrogen ion + Ureidoacrylate peracid + Flavin Mononucleotide Alkanesulfonate + FMNH + Oxygen <> Aldehyde + Flavin Mononucleotide + Sulfite + Water Uracil + FMNH + Oxygen + Thymine <> Ureidoacrylate peracid + Flavin Mononucleotide + (Z)-2-Methyl-ureidoacrylate peracid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||