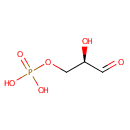
D-Glyceraldehyde 3-phosphate (PAMDB000257)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000257 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | D-Glyceraldehyde 3-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Glyceraldehyde 3-phosphate (G3P) or triose phosphate is an aldotriose, an important metabolic intermediate in both glycolysis and gluconeogenesis, and in tryptophan biosynthesis. G3P is formed from Fructose-1,6-bisphosphate, Dihydroxyacetone phosphate (DHAP),and 1,3-bisphosphoglycerate, (1,3BPG), and this is how glycerol (as DHAP) enters the glycolytic and gluconeogenesis pathways. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C3H7O6P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 170.0578 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 169.998024468 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | LXJXRIRHZLFYRP-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C3H7O6P/c4-1-3(5)2-9-10(6,7)8/h1,3,5H,2H2,(H2,6,7,8) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 142-10-9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | [(2R)-2-hydroxy-3-oxopropoxy]phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | glyceraldehyde-3-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | OC(COP(O)(O)=O)C=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as glyceraldehyde-3-phosphates. These are compounds containing a glyceraldehyde substituted at position O3 by a phosphate group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organooxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carbohydrates and carbohydrate conjugates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Monosaccharides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Glyceraldehyde-3-phosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | D-Tagatose 1,6-bisphosphate <> Dihydroxyacetone phosphate + D-Glyceraldehyde 3-phosphate Fructose 6-phosphate <> Dihydroxyacetone + D-Glyceraldehyde 3-phosphate Indoleglycerol phosphate + L-Serine > D-Glyceraldehyde 3-phosphate + Water + L-Tryptophan Indoleglycerol phosphate > D-Glyceraldehyde 3-phosphate + Indole Fructose 1,6-bisphosphate <> Dihydroxyacetone phosphate + D-Glyceraldehyde 3-phosphate D-Glyceraldehyde 3-phosphate + D-Sedoheptulose 7-phosphate <> D-Erythrose 4-phosphate + Fructose 6-phosphate D-Ribose-5-phosphate + Xylulose 5-phosphate <> D-Glyceraldehyde 3-phosphate + D-Sedoheptulose 7-phosphate D-Glyceraldehyde 3-phosphate + Hydrogen ion + Pyruvic acid <> Carbon dioxide + 1-Deoxy-D-xylulose 5-phosphate D-Glyceraldehyde 3-phosphate + NAD + Phosphate <> Glyceric acid 1,3-biphosphate + Hydrogen ion + NADH + 3-phospho-D-glyceroyl phosphate 2-Keto-3-deoxy-6-phosphogluconic acid <> D-Glyceraldehyde 3-phosphate + Pyruvic acid Dihydroxyacetone phosphate <> D-Glyceraldehyde 3-phosphate D-Glyceraldehyde 3-phosphate + Phosphate + NAD <> Glyceric acid 1,3-biphosphate + NADH + Hydrogen ion beta-D-Fructose 1,6-bisphosphate <> Dihydroxyacetone phosphate + D-Glyceraldehyde 3-phosphate Sedoheptulose 7-phosphate + D-Glyceraldehyde 3-phosphate + D-Sedoheptulose 7-phosphate <> D-Ribose-5-phosphate + Xylulose 5-phosphate Sedoheptulose 7-phosphate + D-Glyceraldehyde 3-phosphate <> D-Erythrose 4-phosphate + beta-D-Fructose 6-phosphate beta-D-Fructose 6-phosphate + D-Glyceraldehyde 3-phosphate <> D-Erythrose 4-phosphate + Xylulose 5-phosphate Pyruvic acid + D-Glyceraldehyde 3-phosphate <> 1-Deoxy-D-xylulose 5-phosphate + Carbon dioxide More...D-Erythrose 4-phosphate + Xylulose 5-phosphate <> Fructose 6-phosphate + D-Glyceraldehyde 3-phosphate 2-Dehydro-3-deoxy-D-galactonate-6-phosphate <> D-Glyceraldehyde 3-phosphate + Pyruvic acid Deoxyribose 5-phosphate <> Acetaldehyde + D-Glyceraldehyde 3-phosphate 2-Dehydro-3-deoxy-D-galactonate 6-phosphate > Pyruvic acid + D-Glyceraldehyde 3-phosphate D-Glyceraldehyde 3-phosphate + Inorganic phosphate + NAD > 3-phospho-D-glyceroyl phosphate + NADH L-Serine + Indoleglycerol phosphate + Indole <> L-Tryptophan + D-Glyceraldehyde 3-phosphate + Water D-Glyceraldehyde 3-phosphate + Pyruvic acid + Hydrogen ion + D-Glyceraldehyde 3-phosphate > 1-Deoxy-D-xylulose 5-phosphate + Carbon dioxide + 1-Deoxy-D-xylulose 5-phosphate D-Glyceraldehyde 3-phosphate + Hydrogen ion + D-Glyceraldehyde 3-phosphate > Carbon dioxide + 1-Deoxy-D-xylulose 5-phosphate + 1-Deoxy-D-xylulose 5-phosphate Xylulose 5-phosphate + D-Ribose-5-phosphate + Xylulose 5-phosphate <> D-Sedoheptulose 7-phosphate + D-Glyceraldehyde 3-phosphate + D-Sedoheptulose 7-phosphate + D-Glyceraldehyde 3-phosphate D-Sedoheptulose 7-phosphate + D-Glyceraldehyde 3-phosphate + D-Sedoheptulose 7-phosphate + D-Glyceraldehyde 3-phosphate <> beta-D-Fructose 6-phosphate + D-Erythrose 4-phosphate Fructose 1,6-bisphosphate + Fructose 1,6-bisphosphate <> D-Glyceraldehyde 3-phosphate + Dihydroxyacetone phosphate + D-Glyceraldehyde 3-phosphate D-Glyceraldehyde 3-phosphate + NAD + Phosphate + D-Glyceraldehyde 3-phosphate > Glyceric acid 1,3-biphosphate + NADH + Hydrogen ion + Glyceric acid 1,3-biphosphate (1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate + (1S,2R)-1-C-(indol-3-yl)glycerol 3-phosphate > D-Glyceraldehyde 3-phosphate + Indole + D-Glyceraldehyde 3-phosphate D-Glyceraldehyde 3-phosphate + D-Glyceraldehyde 3-phosphate <> Dihydroxyacetone phosphate 2-dehydro-3-deoxy-D-galactonate 6-phosphate + 2-Dehydro-3-deoxy-D-galactonate 6-phosphate > Pyruvic acid + D-Glyceraldehyde 3-phosphate + D-Glyceraldehyde 3-phosphate D-tagatofuranose 1,6-bisphosphate > Dihydroxyacetone phosphate + D-Glyceraldehyde 3-phosphate + D-Glyceraldehyde 3-phosphate 2-Keto-3-deoxy-6-phosphogluconic acid > D-Glyceraldehyde 3-phosphate + Pyruvic acid + D-Glyceraldehyde 3-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Ballou, Clinton E.; Fischer, Hermann O. L. The synthesis of D-glyceraldehyde 3-phosphate. Journal of the American Chemical Society (1955), 77 3329-31. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||