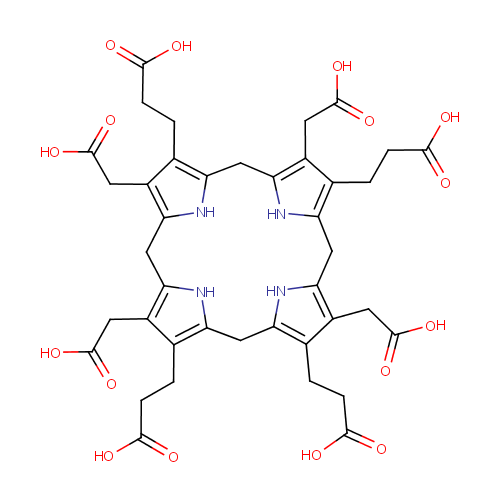
Uroporphyrinogen III (PAMDB000248)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000248 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Uroporphyrinogen III | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Uroporphyrinogens are porphyrinogen variants in which each pyrrole ring has one acetate side chain and one propionate side chain; it is formed by condensation 4 four molecules of porphobilinogen. 4 isomers are possible but only 2 commoly are found, types I and III. Uroporphyrinogen III is a functional intermediate in heme biosynthesis while Uroporphyrinogen I is produced in an abortive side reaction. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C40H44N4O16 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 836.7946 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 836.27523138 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | HUHWZXWWOFSFKF-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C40H44N4O16/c45-33(46)5-1-17-21(9-37(53)54)29-14-27-19(3-7-35(49)50)22(10-38(55)56)30(43-27)15-28-20(4-8-36(51)52)24(12-40(59)60)32(44-28)16-31-23(11-39(57)58)18(2-6-34(47)48)26(42-31)13-25(17)41-29/h41-44H,1-16H2,(H,45,46)(H,47,48)(H,49,50)(H,51,52)(H,53,54)(H,55,56)(H,57,58)(H,59,60) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 1976-85-8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 3-[9,14,20-tris(2-carboxyethyl)-5,10,15,19-tetrakis(carboxymethyl)-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(20),3,5,8,10,13,15,18-octaen-4-yl]propanoic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | uroporphyrinogen-III | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | OC(=O)CCC1=C2CC3=C(CCC(O)=O)C(CC(O)=O)=C(CC4=C(CC(O)=O)C(CCC(O)=O)=C(CC5=C(CC(O)=O)C(CCC(O)=O)=C(CC(N2)=C1CC(O)=O)N5)N4)N3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as porphyrins. These are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Tetrapyrroles and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Porphyrins | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Porphyrins | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 2 S-Adenosylmethionine + Uroporphyrinogen III >2 S-Adenosylhomocysteine + Precorrin 2 + Hydrogen ion Hydroxymethylbilane <> Water + Uroporphyrinogen III 4 Hydrogen ion + Uroporphyrinogen III <>4 Carbon dioxide + Coproporphyrin III 2 S-Adenosylmethionine + Uroporphyrinogen III <>2 S-Adenosylhomocysteine + Precorrin 2 Uroporphyrinogen III <> Coproporphyrin III +4 Carbon dioxide S-Adenosylmethionine + Uroporphyrinogen III <> S-Adenosylhomocysteine + precorrin-1 + Hydrogen ion Hydrogen ion + Uroporphyrinogen III > Carbon dioxide + Coproporphyrinogen III Uroporphyrinogen III > Coproporphyrinogen III +4 Carbon dioxide 2 S-Adenosylmethionine + Uroporphyrinogen III + Precorrin-1 <>2 S-Adenosylhomocysteine + Precorrin 2 Uroporphyrinogen III + S-adenosyl-L-methionine > Hydrogen ion + S-Adenosylhomocysteine + Precorrin-1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||