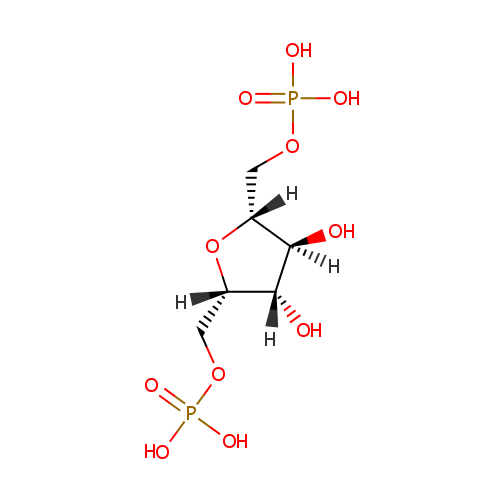
Fructose 1,6-bisphosphate (PAMDB000236)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000236 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Fructose 1,6-bisphosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | The hydrolysis of fructose 1,6-bisphosphate is catalized by Fructose-1,6-bisphosphatase (fru-1,6-P2ase, EC 3.1.3.11) to fructose 6-phosphate and inorganic phosphate and provides a mechanism to permit the reversal of the glycolysis reaction (catalyzed by 6-phosphofructo-1-kinase).(OMIM) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C6H14O12P2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 340.1157 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 339.996048936 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | RNBGYGVWRKECFJ-ZXXMMSQZSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C6H14O12P2/c7-4-3(1-16-19(10,11)12)18-6(9,5(4)8)2-17-20(13,14)15/h3-5,7-9H,1-2H2,(H2,10,11,12)(H2,13,14,15)/t3-,4-,5+,6+/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 488-69-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(2S,3S,4S,5R)-3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]methoxy}phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | [(2S,3S,4S,5R)-3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]methoxyphosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | O[C@H]1[C@H](O)[C@](O)(COP(O)(O)=O)O[C@@H]1COP(O)(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as c-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a C-glycosidic bond. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organooxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carbohydrates and carbohydrate conjugates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Glycosyl compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | C-glycosyl compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Fructose 1,6-bisphosphate <> Dihydroxyacetone phosphate + D-Glyceraldehyde 3-phosphate Adenosine triphosphate + Fructose 6-phosphate > ADP + Fructose 1,6-bisphosphate + Hydrogen ion Fructose 1,6-bisphosphate + Water <> Fructose 6-phosphate + Phosphate Adenosine triphosphate + Fructose 1-phosphate > ADP + Fructose 1,6-bisphosphate + Hydrogen ion Fructose 1,6-bisphosphate + Water > Fructose 6-phosphate + Inorganic phosphate Adenosine triphosphate + Fructose 1-phosphate > ADP + Fructose 1,6-bisphosphate Adenosine triphosphate + Fructose 6-phosphate > ADP + Fructose 1,6-bisphosphate Fructose 6-phosphate + Phosphate + Fructose 6-phosphate > Fructose 1,6-bisphosphate + Water + Fructose 1,6-bisphosphate Fructose 6-phosphate + Adenosine triphosphate + Fructose 6-phosphate > Fructose 1,6-bisphosphate + Adenosine diphosphate + Hydrogen ion + Fructose 1,6-bisphosphate + ADP Fructose 1,6-bisphosphate + Fructose 1,6-bisphosphate <> D-Glyceraldehyde 3-phosphate + Dihydroxyacetone phosphate + D-Glyceraldehyde 3-phosphate Fructose 1,6-bisphosphate + Water + Fructose 1,6-bisphosphate > Phosphate + D-tagatofuranose 6-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Yan, Weiqun. Method for producing fructose-1,6-diphosphate (FDP). Faming Zhuanli Shenqing Gongkai Shuomingshu (2005), 7 pp. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in phosphoric ester hydrolase activity
- Specific function:
- D-fructose 1,6-bisphosphate + H(2)O = D- fructose 6-phosphate + phosphate
- Gene Name:
- fbp
- Locus Tag:
- PA5110
- Molecular weight:
- 37.2 kDa
Reactions
| D-fructose 1,6-bisphosphate + H(2)O = D-fructose 6-phosphate + phosphate. |
- General function:
- Involved in 1-phosphofructokinase activity
- Specific function:
- ATP + D-fructose 1-phosphate = ADP + D- fructose 1,6-bisphosphate
- Gene Name:
- fruK
- Locus Tag:
- PA3561
- Molecular weight:
- 33.4 kDa
Reactions
| ATP + D-fructose 1-phosphate = ADP + D-fructose 1,6-bisphosphate. |