|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000234 |
|---|
|
Identification |
|---|
| Name: |
(S)-3-Hydroxyisobutyryl-CoA |
|---|
| Description: | (S)-3-Hydroxyisobutyryl-CoA is s metabolite of 3-hydroxyisobutyryl-CoA hydrolase (EC 3.1.2.4 ) during beta-alanine metabolism (KEGG 00410), propanoate metabolism (KEGG 00640), and valine, leucine and isoleucine degradation (KEGG 00280). Deficiencies of this enzyme in valine degradation can result in hypotonia, poor feeding, motor delay, and subsequent neurological regression in infancy, episodes of ketoacidosis and Leigh-like changes in the basal ganglia on a magnetic resonance imaging scan (PMID 17160907). |
|---|
|
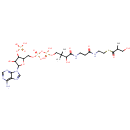
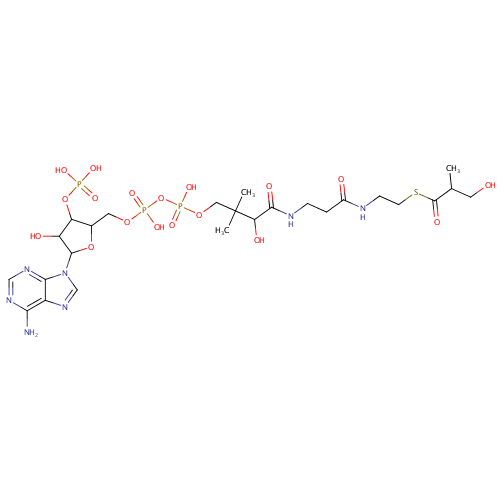
Structure |
|
|---|
| Synonyms: | - (S)-3-Hydroxyisobutyryl-CoA
- (S)-3-Hydroxyisobutyryl-Coenzyme A
- 3-Hydroxy-2-methylpropanoyl-CoA
- 3-Hydroxy-2-methylpropanoyl-Coenzyme A
- 3-Hydroxy-2-methylpropionyl-CoA
- 3-Hydroxy-2-methylpropionyl-Coenzyme A
- 3-Hydroxy-isobutyryl-CoA
- 3-Hydroxy-isobutyryl-coenzyme A
- 3-Hydroxyisobutyryl-CoA
- 3-Hydroxyisobutyryl-Coenzyme A
|
|---|
|
Chemical Formula: |
C25H42N7O18P3S |
|---|
| Average Molecular Weight: |
853.623 |
|---|
| Monoisotopic Molecular
Weight: |
853.151987801 |
|---|
| InChI Key: |
WWEOGFZEFHPUAM-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C25H42N7O18P3S/c1-13(8-33)24(38)54-7-6-27-15(34)4-5-28-22(37)19(36)25(2,3)10-47-53(44,45)50-52(42,43)46-9-14-18(49-51(39,40)41)17(35)23(48-14)32-12-31-16-20(26)29-11-30-21(16)32/h11-14,17-19,23,33,35-36H,4-10H2,1-3H3,(H,27,34)(H,28,37)(H,42,43)(H,44,45)(H2,26,29,30)(H2,39,40,41) |
|---|
| CAS
number: |
319440-43-2 |
|---|
| IUPAC Name: | {[5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({[hydroxy(3-hydroxy-3-{[2-({2-[(3-hydroxy-2-methylpropanoyl)sulfanyl]ethyl}carbamoyl)ethyl]carbamoyl}-2,2-dimethylpropoxy)phosphoryl]oxy})phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid |
|---|
|
Traditional IUPAC Name: |
[5-(6-aminopurin-9-yl)-4-hydroxy-2-[({hydroxy[hydroxy(3-hydroxy-3-{[2-({2-[(3-hydroxy-2-methylpropanoyl)sulfanyl]ethyl}carbamoyl)ethyl]carbamoyl}-2,2-dimethylpropoxy)phosphoryl]oxyphosphoryl}oxy)methyl]oxolan-3-yl]oxyphosphonic acid |
|---|
| SMILES: | CC(CO)C(=O)SCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(O)(=O)OP(O)(=O)OCC1OC(C(O)C1OP(O)(O)=O)N1C=NC2=C(N)N=CN=C12 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as acyl coas. These are organic compounds containing a coenzyme A substructure linked to an acyl chain. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
|
Direct Parent |
Acyl CoAs |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Coenzyme a or derivatives
- Purine ribonucleoside diphosphate
- Purine ribonucleoside 3',5'-bisphosphate
- N-glycosyl compound
- Glycosyl compound
- Beta amino acid or derivatives
- Organic pyrophosphate
- Monosaccharide phosphate
- 6-aminopurine
- Purine
- Imidazopyrimidine
- Monoalkyl phosphate
- Aminopyrimidine
- Imidolactam
- Alkyl phosphate
- Pyrimidine
- Primary aromatic amine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- N-substituted imidazole
- N-acyl-amine
- Monosaccharide
- Fatty amide
- Saccharide
- Heteroaromatic compound
- Oxolane
- Imidazole
- Azole
- Thiocarboxylic acid ester
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Sulfenyl compound
- Thioether
- Thiocarboxylic acid or derivatives
- Carboxylic acid derivative
- Carboxylic acid amide
- Hydrocarbon derivative
- Primary amine
- Primary alcohol
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Valine, leucine and isoleucine degradation pae00280
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Lehnert W, Sass JO: Glutaconyl-CoA is the main toxic agent in glutaryl-CoA dehydrogenase deficiency (glutaric aciduria type I). Med Hypotheses. 2005;65(2):330-3. Pubmed: 15922108
- Loupatty, F. J., Clayton, P. T., Ruiter, J. P., Ofman, R., Ijlst, L., Brown, G. K., Thorburn, D. R., Harris, R. A., Duran, M., Desousa, C., Krywawych, S., Heales, S. J., Wanders, R. J. (2007). "Mutations in the gene encoding 3-hydroxyisobutyryl-CoA hydrolase results in progressive infantile neurodegeneration." Am J Hum Genet 80:195-199. Pubmed: 17160907
|
|---|
| Synthesis Reference: |
Hawes, John W.; Harper, Edwin T. Synthesis of methacrylyl-CoA and (R)- and (S)-3-hydroxyisobutyryl-CoA. Methods in Enzymology (2000), 324(Branched-Chain Amino Acids), 73-79. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|