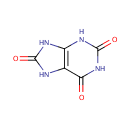
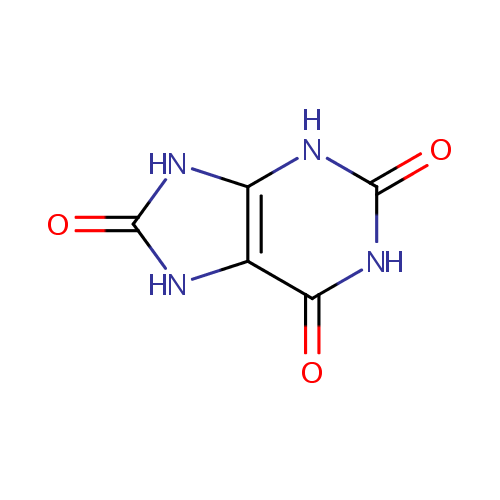
| References: |
- Alderman M, Aiyer KJ: Uric acid: role in cardiovascular disease and effects of losartan. Curr Med Res Opin. 2004 Mar;20(3):369-79. Pubmed: 15025846
- Cacabelos R, Fernandez-Novoa L, Corzo L, Pichel V, Lombardi V, Kubota Y: Genomics and phenotypic profiles in dementia: implications for pharmacological treatment. Methods Find Exp Clin Pharmacol. 2004 Jul-Aug;26(6):421-44. Pubmed: 15349138
- Eells JT, Spector R: Purine and pyrimidine base and nucleoside concentrations in human cerebrospinal fluid and plasma. Neurochem Res. 1983 Nov;8(11):1451-7. Pubmed: 6656991
- Hanvivadhanakul P, Akkasilpa S, Deesomchok U: Efficacy of benzbromarone compared to allopurinol in lowering serum uric acid level in hyperuricemic patients. J Med Assoc Thai. 2002 Jun;85 Suppl 1:S40-7. Pubmed: 12188443
- Inoue K, Namiki T, Iwasaki Y, Yoshimura Y, Nakazawa H: Determination of uric acid in human saliva by high-performance liquid chromatography with amperometric electrochemical detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Feb 25;785(1):57-63. Pubmed: 12535838
- Iwai N, Mino Y, Hosoyamada M, Tago N, Kokubo Y, Endou H: A high prevalence of renal hypouricemia caused by inactive SLC22A12 in Japanese. Kidney Int. 2004 Sep;66(3):935-44. Pubmed: 15327384
- Kanbay M, Akcay A, Huddam B, Usluogullari CA, Arat Z, Ozdemir FN, Haberal M: Influence of cyclosporine and tacrolimus on serum uric acid levels in stable kidney transplant recipients. Transplant Proc. 2005 Sep;37(7):3119-20. Pubmed: 16213325
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Kastenbauer S, Koedel U, Becker BF, Pfister HW: Oxidative stress in bacterial meningitis in humans. Neurology. 2002 Jan 22;58(2):186-91. Pubmed: 11805243
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Kirschbaum B: Correlation studies of plasma paraoxonase activity and uric acid concentration with AAPH-Induced erythrocyte hemolysis in hemodialysis patients. Artif Organs. 2004 Mar;28(3):259-64. Pubmed: 15046624
- Marinaki AM, Champion M, Kurian MA, Simmonds HA, Marie S, Vincent MF, van den Berghe G, Duley JA, Fairbanks LD: Adenylosuccinate lyase deficiency--first British case. Nucleosides Nucleotides Nucleic Acids. 2004 Oct;23(8-9):1231-3. Pubmed: 15571235
- Mazzali M: Uric acid and transplantation. Semin Nephrol. 2005 Jan;25(1):50-5. Pubmed: 15660335
- Puig JG, Torres R, Ruilope LM: AT1 blockers and uric acid metabolism: are there relevant differences? J Hypertens Suppl. 2002 Jun;20(5):S29-31. Pubmed: 12184060
- Simkin PA, Hoover PL, Paxson CS, Wilson WF: Uric acid excretion: quantitative assessment from spot, midmorning serum and urine samples. Ann Intern Med. 1979 Jul;91(1):44-7. Pubmed: 464453
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. Pubmed: 19212411
- Srinivasan S, Kalaiselvi P, Sakthivel R, Pragasam V, Muthu V, Varalakshmi P: Uric acid: an abettor or protector in calcium oxalate urolithiasis? Biochemical study in stone formers. Clin Chim Acta. 2005 Mar;353(1-2):45-51. Pubmed: 15698589
- Sysyn GD, Rozycki HJ: Lack of prognostic significance of early elevated serum uric acid levels in low birthweight infants. Biol Neonate. 2003;83(4):253-7. Pubmed: 12743454
- Tumgor G, Arikan C, Kilic M, Aydogdu S: Frequency of hyperuricemia and effect of calcineurin inhibitors on serum uric acid levels in liver transplanted children. Pediatr Transplant. 2006 Sep;10(6):665-8. Pubmed: 16911488
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Williams KP, Galerneau F: The role of serum uric acid as a prognostic indicator of the severity of maternal and fetal complications in hypertensive pregnancies. J Obstet Gynaecol Can. 2002 Aug;24(8):628-32. Pubmed: 12196841
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|