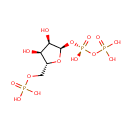
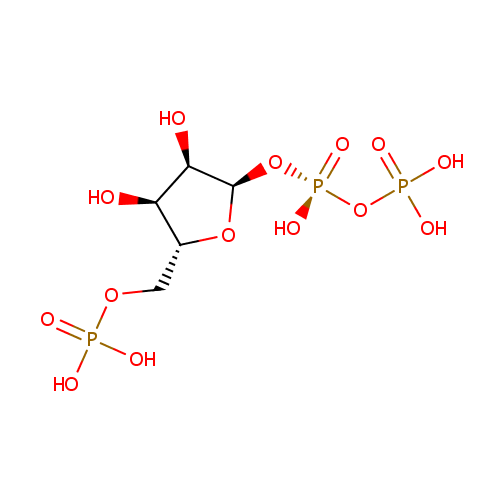
Phosphoribosyl pyrophosphate (PAMDB000115)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000115 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Phosphoribosyl pyrophosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Phosphoribosyl pyrophosphate (PRPP) is a pentosephosphate. The key substance in the biosynthesis of histidine, tryptophan, and purine and pyrimidine nucleotides. It is formed from ribose 5-phosphate by the enzyme ribose-phosphate diphosphokinase. It plays a role in transferring phosphate groups in several reactions. (Wikipedia) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C5H13O14P3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 390.0696 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 389.95181466 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | PQGCEDQWHSBAJP-TXICZTDVSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C5H13O14P3/c6-3-2(1-16-20(8,9)10)17-5(4(3)7)18-22(14,15)19-21(11,12)13/h2-7H,1H2,(H,14,15)(H2,8,9,10)(H2,11,12,13)/t2-,3-,4-,5-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 7540-64-9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | [({[(2R,3R,4S,5R)-3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]oxy}(hydroxy)phosphoryl)oxy]phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | phosphoribosylpyrophosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | O[C@H]1[C@@H](O)[C@@H](O[P@](O)(=O)OP(O)(O)=O)O[C@@H]1COP(O)(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as monosaccharide phosphates. These are monosaccharides comprising a phosphated group linked to the carbohydrate unit. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organooxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carbohydrates and carbohydrate conjugates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Monosaccharides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Monosaccharide phosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Guanine + Phosphoribosyl pyrophosphate > Guanosine monophosphate + Pyrophosphate Hypoxanthine + Phosphoribosyl pyrophosphate <> Inosinic acid + Pyrophosphate 2 Hydrogen ion + Phosphoribosyl pyrophosphate + Quinolinic acid <> Carbon dioxide + Nicotinamide ribotide + Pyrophosphate Phosphoribosyl pyrophosphate + Xanthine <> Pyrophosphate + Xanthylic acid Adenine + Phosphoribosyl pyrophosphate <> Adenosine monophosphate + Pyrophosphate Adenosine triphosphate + Water + Nicotinic acid + Phosphoribosyl pyrophosphate > ADP + Nicotinamide ribotide + Phosphate + Pyrophosphate Adenosine triphosphate + Phosphoribosyl pyrophosphate <> Pyrophosphate + Phosphoribosyl-ATP Phosphoribosyl pyrophosphate + Uracil <> Pyrophosphate + Uridine 5'-monophosphate Orotidylic acid + Pyrophosphate <> Orotic acid + Phosphoribosyl pyrophosphate Adenosine triphosphate + Ribose 1,5-bisphosphate <> ADP + Phosphoribosyl pyrophosphate Adenosine triphosphate + D-Ribose-5-phosphate <> Adenosine monophosphate + Phosphoribosyl pyrophosphate Nicotinamide ribotide + Pyrophosphate + Carbon dioxide <> Quinolinic acid + Phosphoribosyl pyrophosphate AICAR + Pyrophosphate <> 5-Amino-4-imidazolecarboxyamide + Phosphoribosyl pyrophosphate More...6-Mercaptopurine + Phosphoribosyl pyrophosphate <> 6-Thioinosine-5'-monophosphate + Pyrophosphate 6-Methylmercaptopurine + Phosphoribosyl pyrophosphate <> 6-Methylthiopurine 5'-monophosphate ribonucleotide + Pyrophosphate Thioguanine + Phosphoribosyl pyrophosphate <> 6-Thioguanosine monophosphate + Pyrophosphate Nicotinamide ribotide + Pyrophosphate < Hydrogen ion + Nicotinic acid + Phosphoribosyl pyrophosphate 5-Phosphoribosylamine + Pyrophosphate + L-Glutamate < Phosphoribosyl pyrophosphate + L-Glutamine + Water Adenosine triphosphate + D-Ribose-5-phosphate <> Hydrogen ion + Phosphoribosyl pyrophosphate + Adenosine monophosphate N-(5-Phospho-D-ribosyl)anthranilate + Pyrophosphate < 2-Aminobenzoic acid + Phosphoribosyl pyrophosphate Nicotinamide ribotide + Pyrophosphate > Nicotinic acid + Phosphoribosyl pyrophosphate Phosphoribosyl pyrophosphate + Hydrogen ion > Pyrophosphate + Phosphoribosyl-ATP + Phosphoribosyl-ATP 2-Aminobenzoic acid + Phosphoribosyl pyrophosphate > Pyrophosphate + N-(5-phosphoribosyl)-anthranilate + N-(5-phosphoribosyl)-anthranilate Quinolinic acid + Hydrogen ion + Phosphoribosyl pyrophosphate > Carbon dioxide + Pyrophosphate + nicotinate beta-D-ribonucleotide + Nicotinamide ribotide Nicotinic acid + Water + Adenosine triphosphate + Phosphoribosyl pyrophosphate > Phosphate + Adenosine diphosphate + Pyrophosphate + nicotinate beta-D-ribonucleotide + ADP + Nicotinamide ribotide Ribose 1,5-bisphosphate + Adenosine triphosphate + Ribose 1,5-bisphosphate > Adenosine diphosphate + Phosphoribosyl pyrophosphate + ADP Phosphoribosyl pyrophosphate + Water + L-Glutamine > 5-Phosphoribosylamine + L-Glutamic acid + Pyrophosphate + 5-Phosphoribosylamine + L-Glutamate Orotic acid + Phosphoribosyl pyrophosphate > Pyrophosphate + Orotidylic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Gross, Akiva; Abril, Obsidiana; Lewis, Jerome M.; Geresh, Shimona; Whitesides, George M. Practical synthesis of 5-phospho-D-ribosyl a-1-pyrophosphate (PRPP): enzymatic routes from ribose 5-phosphate or ribose. Journal of the American Chemical Society ( | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||