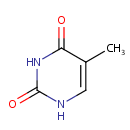
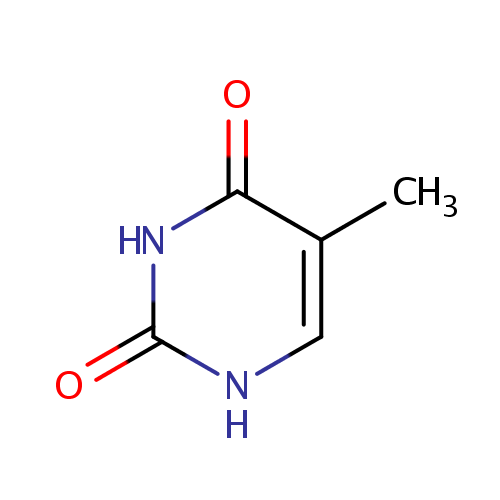
Thymine (PAMDB000110)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000110 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Thymine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Thymine is a nucleobase that is also known as 5-methyluracil. As the name suggests, thymine may be derived by methylation of uracil at the 5th carbon. In RNA, thymine is replaced with uracil. In DNA, thymine (T) binds to adenine (A) via two hydrogen bonds, thus stabilizing the nucleic acid structures. Thymine combined with deoxyribose creates the nucleoside deoxythymidine, when it is combined with ribose it creates the nucleoside thymidine. Thymidine can be phosphorylated with one, two, or three phosphoric acid groups, creating, respectively, TMP, TDP, or TTP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C5H6N2O2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 126.1133 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 126.042927446 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | RWQNBRDOKXIBIV-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C5H6N2O2/c1-3-2-6-5(9)7-4(3)8/h2H,1H3,(H2,6,7,8,9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 65-71-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 5-methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | thymine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC1=CNC(=O)NC1=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as pyrimidones. These are compounds that contain a pyrimidine ring,which bears a ketone. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Diazines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyrimidines and pyrimidine derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyrimidones | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 320 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Phosphate + Thymidine <> Deoxyribose 1-phosphate + Thymine 5-Methylcytosine + Water <> Thymine + Ammonia Thymine + Oxygen + FMNH > (<i>Z</i>)-2-methylureidoacrylate peracid + Flavin Mononucleotide + Hydrogen ion Dihydrothymine + NAD <> Thymine + NADH + Hydrogen ion Thymidine + Phosphate <> deoxyribose-1-phosphate + Thymine Dihydrothymine + NAD > Thymine + NADH Thymine + FMNH(2) + Oxygen > (Z)-2-Methyl-ureidoacrylate peracid + Flavin Mononucleotide + Water Dihydrouracil + NAD + Dihydrothymine <> Uracil + NADH + Hydrogen ion + Thymine Uracil + FMNH + Oxygen + Thymine <> Ureidoacrylate peracid + Flavin Mononucleotide + (Z)-2-Methyl-ureidoacrylate peracid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Zhang, Shi-Ying; Wu, Da-Jun; Zhang, Yan-Ping. Synthesis of thymine. Zhongguo Yiyao Gongye Zazhi (1999), 30(7), 325. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in hydrolase activity, acting on carbon-nitrogen (but not peptide) bonds
- Specific function:
- Cytosine + H(2)O = uracil + NH(3)
- Gene Name:
- codA
- Locus Tag:
- PA0437
- Molecular weight:
- 47.1 kDa
Reactions
| Cytosine + H(2)O = uracil + NH(3). |