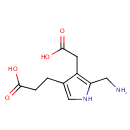
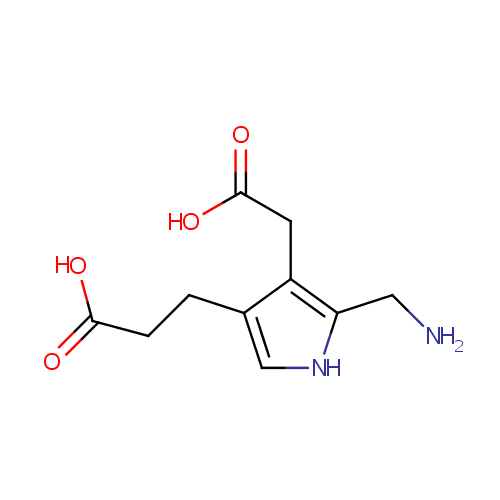
Porphobilinogen (PAMDB000104)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000104 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Porphobilinogen | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Porphobilinogen is a pyrrole involved in porphyrin metabolism. -- Wikipedia; It consists of a pyrrole ring with acetyl, propionyl, and aminomethyl side chains; It is a key monopyrrolic intermediate in porphyrin, chlorophyll and vitamin B12 biosynthesis. Porphobilinogen is generated by the enzyme ALA dehydratase by combining two molecules of dALA together, and converted into hydroxymethyl bilane by the enzyme porphobilinogen deaminase. 4 molecules of porphobilinogen are condensed to form one molecule of uroporphyrinogen I, which is then converted successively to coproporphyrinogen I, protoporphyrin IX, and heme. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C10H14N2O4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 226.2292 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 226.095356946 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | QSHWIQZFGQKFMA-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C10H14N2O4/c11-4-8-7(3-10(15)16)6(5-12-8)1-2-9(13)14/h5,12H,1-4,11H2,(H,13,14)(H,15,16) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 487-90-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 3-[5-(aminomethyl)-4-(carboxymethyl)-1H-pyrrol-3-yl]propanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | porphobilinogen | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | NCC1=C(CC(O)=O)C(CCC(O)=O)=CN1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as aralkylamines. These are alkylamines in which the alkyl group is substituted at one carbon atom by an aromatic hydrocarbyl group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organonitrogen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Amines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Aralkylamines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Aralkylamines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Water + 4 Porphobilinogen > Hydroxymethylbilane +4 Ammonium 2 5-Aminolevulinic acid <> Porphobilinogen +2 Water 4 Porphobilinogen + Water <> Hydroxymethylbilane +4 Ammonia Water + Porphobilinogen <> Hydrogen ion + Ammonia + Hydroxymethylbilane 5-Aminolevulinic acid <> Hydrogen ion + Water + Porphobilinogen | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Frydman, Benjamin; Despuy, Maria E.; Rapoport, Henry. Pyrroles from azaindoles. A synthesis of porphobilinogen. Journal of the American Chemical Society (1965), 87(15), 3530-1. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||