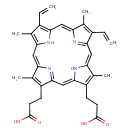
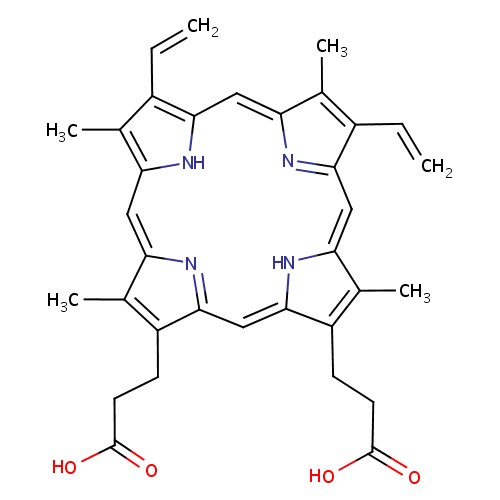
Protoporphyrin IX (PAMDB000101)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000101 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Protoporphyrin IX | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Protoporphyrins are tetrapyrroles containing 4 methyl, 2 propionic and 2 vinyl side chains. Protopophyrin is produced by oxidation of the methylene bridge of protoporphyrinogen. Protoporphyrin IX is the only naturally occurring isomer; it is an intermediate in heme biosynthesis, combining with ferrous iron to form protoheme IX, the heme prosthetic group of hemoglobin. Protoporphyrin IX is created by the enzyme protoporphyrinogen oxidase. The enzyme ferrochelatase converts it into heme. Protoporphyrin IX naturally occurs in small amounts in feces. It is accumulated and excreted excessively in the feces in protoporphyria and variegate porphyria. Protoporphyrin IX is also responsible for the brown pigment (Ooporphyrin) of birds' eggs Protoporphyrin IX is used as a branch point in the biosynthetic pathway leading to Heme (by insertion of iron) and chlorophylls (by insertion of Mg and further side-chain transformation). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C34H34N4O4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 562.6582 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 562.258005596 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | KSFOVUSSGSKXFI-UJJXFSCMSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C34H34N4O4/c1-7-21-17(3)25-13-26-19(5)23(9-11-33(39)40)31(37-26)16-32-24(10-12-34(41)42)20(6)28(38-32)15-30-22(8-2)18(4)27(36-30)14-29(21)35-25/h7-8,13-16,35,38H,1-2,9-12H2,3-6H3,(H,39,40)(H,41,42)/b25-13-,26-13-,27-14-,28-15-,29-14-,30-15-,31-16-,32-16- | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 553-12-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 3-[20-(2-carboxyethyl)-9,14-diethenyl-5,10,15,19-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(21),2,4,6,8(23),9,11,13,15,17,19-undecaen-4-yl]propanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | protoporphyrin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC1=C(CCC(O)=O)/C2=C/C3=N/C(=C\C4=C(C)C(C=C)=C(N4)/C=C4\N=C(\C=C\1/N\2)C(C=C)=C4C)/C(C)=C3CCC(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as porphyrins. These are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Tetrapyrroles and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Porphyrins | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Porphyrins | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 300 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Membrane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Oxygen + Protoporphyrinogen IX >3 Water + Protoporphyrin IX 3 Fumaric acid + Protoporphyrinogen IX > Protoporphyrin IX +3 Succinic acid Protoporphyrin IX + Fe2+ <> Heme +2 Hydrogen ion + Fe2+ Protoporphyrinogen IX + 3 Menaquinone + 3 Menaquinone <> Protoporphyrin IX +3 Menaquinol 6 Iron + Protoporphyrin IX > Heme + Hydrogen ion Protoporphyrinogen IX + Oxygen > Protoporphyrin IX + Hydrogen peroxide Protoporphyrinogen IX + a menaquinone > Protoporphyrin IX + a menaquinol Protoporphyrinogen IX + 3 Menaquinone > Protoporphyrin IX +3 Menaquinol 6 Protoporphyrinogen IX + menaquinone-8 > Protoporphyrin IX + Menaquinol 6 Protoporphyrin IX + Iron >2 Hydrogen ion + ferroheme b | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Games, David E.; Jackson, Anthony H.; Jackson, J. Richard; Belcher, Roderick V.; Smith, Sydney G. Biosynthesis of protoporphyrin-IX from coproporphyrinogen-III. Journal of the Chemical Society, Chemical Communications (1976), (6), 187-8. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||