|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000093 |
|---|
|
Identification |
|---|
| Name: |
Oxoadipic acid |
|---|
| Description: | 2-Oxoadipic acid is produced from lysine in the cytosol of cells via the saccharopine and the pipecolic acid pathways. Catabolites of hydroxylysine and tryptophan enter these pathways as 2-aminoadipic- -semialdehyde and 2-oxoadipate, respectively. In the cytoplasm, 2-oxoadipate is decarboxylated to glutaryl-CoA by the 2-oxoadipate dehydrogenase complex and then converted to acetyl-CoA. |
|---|
|
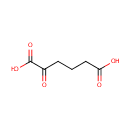
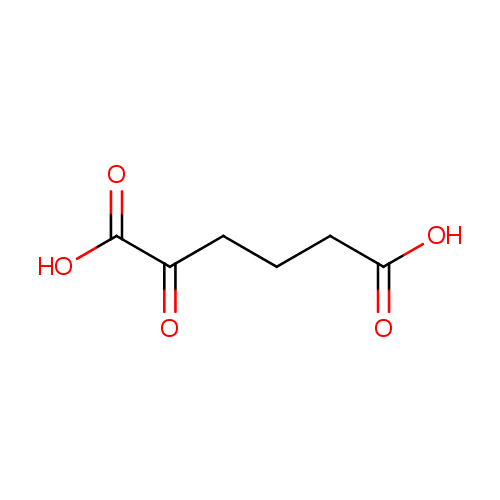
Structure |
|
|---|
| Synonyms: | - α-ketoadipate
- α-ketoadipic acid
- 2-Keto-adipate
- 2-Keto-adipic acid
- 2-Ketoadipate
- 2-Ketoadipic acid
- 2-Oxo-hexanedioate
- 2-Oxo-hexanedioic acid
- 2-Oxoadipate
- 2-Oxoadipic acid
- 2-Oxohexanedioate
- 2-Oxohexanedioic acid
- 2-Oxohexanedionate
- 2-Oxohexanedionic acid
- A-Ketoadipate
- A-Ketoadipic acid
- A-Oxoadipate
- A-Oxoadipic acid
- Alpha-Ketoadipate
- Alpha-Ketoadipic acid
- Alpha-Oxoadipate
- Alpha-Oxoadipic acid
- Oxoadipate
- α-Ketoadipate
- α-Ketoadipic acid
- α-Oxoadipate
- α-Oxoadipic acid
|
|---|
|
Chemical Formula: |
C6H8O5 |
|---|
| Average Molecular Weight: |
160.1247 |
|---|
| Monoisotopic Molecular
Weight: |
160.037173366 |
|---|
| InChI Key: |
FGSBNBBHOZHUBO-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C6H8O5/c7-4(6(10)11)2-1-3-5(8)9/h1-3H2,(H,8,9)(H,10,11) |
|---|
| CAS
number: |
3184-35-8 |
|---|
| IUPAC Name: | 2-oxohexanedioic acid |
|---|
|
Traditional IUPAC Name: |
oxoadipate |
|---|
| SMILES: | OC(=O)CCCC(=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as medium-chain keto acids and derivatives. These are keto acids with a 6 to 12 carbon atoms long side chain. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Keto acids and derivatives |
|---|
| Sub Class | Medium-chain keto acids and derivatives |
|---|
|
Direct Parent |
Medium-chain keto acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Medium-chain keto acid
- Dicarboxylic acid or derivatives
- Alpha-keto acid
- Alpha-hydroxy ketone
- Ketone
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
127 |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Barshop BA, Nyhan WL, Naviaux RK, McGowan KA, Friedlander M, Haas RH: Kearns-Sayre syndrome presenting as 2-oxoadipic aciduria. Mol Genet Metab. 2000 Jan;69(1):64-8. Pubmed: 10655159
- Fiermonte G, Dolce V, Palmieri L, Ventura M, Runswick MJ, Palmieri F, Walker JE: Identification of the human mitochondrial oxodicarboxylate carrier. Bacterial expression, reconstitution, functional characterization, tissue distribution, and chromosomal location. J Biol Chem. 2001 Mar 16;276(11):8225-30. Epub 2000 Nov 16. Pubmed: 11083877
- Guneral F, Bachmann C: Age-related reference values for urinary organic acids in a healthy Turkish pediatric population. Clin Chem. 1994 Jun;40(6):862-6. Pubmed: 8087979
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Lee SH, Kim SO, Chung BC: Gas chromatographic-mass spectrometric determination of urinary oxoacids using O-(2,3,4,5,6-pentafluorobenzyl)oxime-trimethylsilyl ester derivatization and cation-exchange chromatography. J Chromatogr B Biomed Sci Appl. 1998 Nov 20;719(1-2):1-7. Pubmed: 9869358
- Schulze-Bergkamen A, Okun JG, Spiekerkotter U, Lindner M, Haas D, Kohlmuller D, Mayatepek E, Schulze-Bergkamen H, Greenberg CR, Zschocke J, Hoffmann GF, Kolker S: Quantitative acylcarnitine profiling in peripheral blood mononuclear cells using in vitro loading with palmitic and 2-oxoadipic acids: biochemical confirmation of fatty acid oxidation and organic acid disorders. Pediatr Res. 2005 Nov;58(5):873-80. Epub 2005 Sep 23. Pubmed: 16183823
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|
| Synthesis Reference: |
Nelson, Randall B.; Gribble, Gordon W. Preparation of a-ketoadipic acid. Organic Preparations and Procedures International (1973), 5(2), 55-8. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|