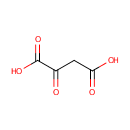
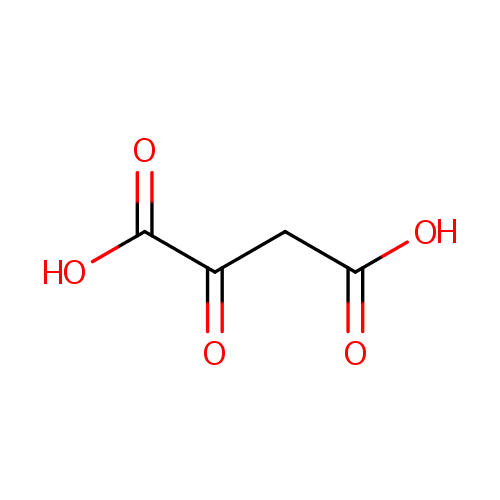
Oxalacetic acid (PAMDB000092)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000092 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Oxalacetic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Oxaloacetic acid, also known as oxosuccinic acid or oxalacetic acid, is a four-carbon dicarboxylic acid appearing as an intermediate of the citric acid cycle. In vivo, oxaloacetate (the ionized form of oxaloacetic acid) is formed by the oxidation of L-malate, catalyzed by malate dehydrogenase, and reacts with Acetyl-CoA to form citrate, catalyzed by citrate synthase.(wikipedia) A class of ketodicarboxylic acids derived from oxalic acid. Oxaloacetic acid is an intermediate in the citric acid cycle and is converted to aspartic acidD by a transamination reaction. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C4H4O5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 132.0716 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 132.005873238 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | KHPXUQMNIQBQEV-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C4H4O5/c5-2(4(8)9)1-3(6)7/h1H2,(H,6,7)(H,8,9) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 328-42-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 2-oxobutanedioic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | oxalacetate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | OC(=O)CC(=O)C(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as short-chain keto acids and derivatives. These are keto acids with an alkyl chain the contains less than 6 carbon atoms. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Keto acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Short-chain keto acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Short-chain keto acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 161 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Citric acid <> Acetic acid + Oxalacetic acid Tartaric acid <> Water + Oxalacetic acid Water + Oxalacetic acid + Propionyl-CoA <> Methylcitric acid + Coenzyme A + Hydrogen ion + (2S,3S)-2-hydroxybutane-1,2,3-tricarboxylate Acetyl-CoA + Water + Oxalacetic acid <> Citric acid + Coenzyme A + Hydrogen ion alpha-Ketoglutarate + L-Aspartic acid <> L-Glutamate + Oxalacetic acid Hydrogen ion + Oxalacetic acid > Carbon dioxide + Pyruvic acid L-Malic acid + Ubiquinone-8 > Oxalacetic acid + Ubiquinol-8 L-Malic acid + Menaquinone 8 > Menaquinol 8 + Oxalacetic acid L-Malic acid + NAD <> Hydrogen ion + NADH + Oxalacetic acid Adenosine triphosphate + Oxalacetic acid <> ADP + Carbon dioxide + Phosphoenolpyruvic acid Carbon dioxide + Water + Phosphoenolpyruvic acid <> Hydrogen ion + Oxalacetic acid + Phosphate + Hydrogen carbonate D-tartrate > Water + Oxalacetic acid Phosphate + Oxalacetic acid <> Water + Phosphoenolpyruvic acid + Carbon dioxide Citric acid + Coenzyme A <> Acetyl-CoA + Water + Oxalacetic acid L-Aspartic acid + Water + Oxygen <> Oxalacetic acid + Ammonia + Hydrogen peroxide Methylcitric acid + Coenzyme A <> Propionyl-CoA + Oxalacetic acid + Water L-Malic acid + FAD <> FADH2 + Oxalacetic acid Oxalacetic acid + Water + Propionyl-CoA <> Hydrogen ion + Methylcitric acid + Coenzyme A L-Aspartic acid + Oxoglutaric acid <> Oxalacetic acid + L-Glutamate L-Malic acid + a quinone > Oxalacetic acid + a quinol More...L-Malic acid + Oxygen <> Oxalacetic acid + Hydrogen peroxide Oxalacetic acid enol-oxaloacetate Phosphate + Oxalacetic acid <> Phosphoenolpyruvic acid + Hydrogen carbonate Pyridoxamine + Oxalacetic acid <> Pyridoxal + L-Aspartic acid Inorganic phosphate + Oxalacetic acid > Water + Phosphoenolpyruvic acid + Carbonic acid (3S)-Citryl-CoA > Acetyl-CoA + Oxalacetic acid L-Malic acid + NAD > Oxalacetic acid + NADH L-Malic acid + a quinone > Oxalacetic acid + reduced quinone L-Malic acid + NAD + Oxalacetic acid <> Pyruvic acid + Carbon dioxide + NADH L-Malic acid + Quinone <> Oxalacetic acid + Hydroquinone L-Malic acid + NADP + Oxalacetic acid <> Pyruvic acid + Carbon dioxide + NADPH L-Malic acid + NAD + L-Malic acid <> Oxalacetic acid + NADH + Hydrogen ion Adenosine triphosphate + Pyruvic acid + Hydrogen carbonate > Adenosine diphosphate + Phosphate + Oxalacetic acid + ADP L-Malic acid + Quinone + L-Malic acid > Oxalacetic acid + Hydroquinone L-Aspartic acid + Oxoglutaric acid + L-Aspartic acid > Oxalacetic acid + L-Glutamic acid + L-Glutamate L-Aspartic acid + Water + Oxygen + L-Aspartic acid > Oxalacetic acid + Ammonia + Hydrogen peroxide Oxalacetic acid + Adenosine triphosphate > Adenosine diphosphate + Carbon dioxide + Phosphoenolpyruvic acid + ADP Propionyl-CoA + Water + Oxalacetic acid + Propionyl-CoA > Coenzyme A + Hydrogen ion + 2-Methylcitric acid + Methylcitric acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Heidelberger, Charles; Hurlbert, Robert B. The synthesis of oxalacetic acid-I-C14 and orotic acid-6-C14. Journal of the American Chemical Society (1950), 72 4704-6. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||