|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000090 |
|---|
|
Identification |
|---|
| Name: |
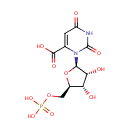
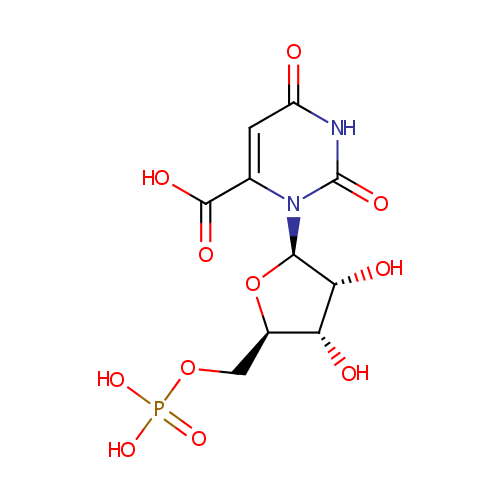
Orotidylic acid |
|---|
| Description: | Orotidylic acid (OMP), is a pyrimidine nucleotide which is the last intermediate in the biosynthesis of uridine monophosphate. Decarboxylation by Orotidylate decarboxylase affords Uridine 5'-phosphate which is the route to Uridine and its derivatives de novo and consequently one of the most important processes in nucleic acid synthesis (Dictionary of Organic Compounds). In Pseudomonas aeruginosa, the enzyme UMP synthase converts OMP into uridine 5'- monophosphate. If UMP synthase is defective, orotic aciduria can result. (Wikipedia) |
|---|
|
Structure |
|
|---|
| Synonyms: | - 1,2,3,6-Tetrahydro-2,6-dioxo-3-(5-O-phosphono-b-D-ribofuranosyl)-4-Pyrimidinecarboxylate
- 1,2,3,6-Tetrahydro-2,6-dioxo-3-(5-O-phosphono-b-D-ribofuranosyl)-4-Pyrimidinecarboxylic acid
- 1,2,3,6-tetrahydro-2,6-dioxo-3-(5-O-phosphono-b-delta-Ribofuranosyl)-4-pyrimidinecarboxylate
- 1,2,3,6-tetrahydro-2,6-dioxo-3-(5-O-phosphono-b-delta-Ribofuranosyl)-4-pyrimidinecarboxylic acid
- 1,2,3,6-tetrahydro-2,6-dioxo-3-(5-O-phosphono-b-δ-Ribofuranosyl)-4-pyrimidinecarboxylate
- 1,2,3,6-tetrahydro-2,6-dioxo-3-(5-O-phosphono-b-δ-Ribofuranosyl)-4-pyrimidinecarboxylic acid
- 1,2,3,6-Tetrahydro-2,6-dioxo-3-(5-O-phosphono-beta-delta-ribofuranosyl)-4-Pyrimidinecarboxylate
- 1,2,3,6-Tetrahydro-2,6-dioxo-3-(5-O-phosphono-beta-delta-ribofuranosyl)-4-Pyrimidinecarboxylic acid
- 1,2,3,6-tetrahydro-2,6-dioxo-3-(5-O-phosphono-β-δ-Ribofuranosyl)-4-pyrimidinecarboxylate
- 1,2,3,6-tetrahydro-2,6-dioxo-3-(5-O-phosphono-β-δ-Ribofuranosyl)-4-pyrimidinecarboxylic acid
- 2,6-dioxo-3-(5-O-phosphono-b-D-Ribofuranosyl)-1,2,3,6-tetrahydropyrimidine-4-carboxylate
- 2,6-dioxo-3-(5-O-phosphono-b-D-Ribofuranosyl)-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid
- 2,6-dioxo-3-(5-O-phosphono-b-delta-Ribofuranosyl)-1,2,3,6-tetrahydropyrimidine-4-carboxylate
- 2,6-dioxo-3-(5-O-phosphono-b-delta-Ribofuranosyl)-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid
- 2,6-dioxo-3-(5-O-phosphono-b-δ-Ribofuranosyl)-1,2,3,6-tetrahydropyrimidine-4-carboxylate
- 2,6-dioxo-3-(5-O-phosphono-b-δ-Ribofuranosyl)-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid
- 2,6-Dioxo-3-(5-O-phosphono-beta-D-ribofuranosyl)-1,2,3,6-tetrahydropyrimidine-4-carboxylate
- 2,6-Dioxo-3-(5-O-phosphono-beta-D-ribofuranosyl)-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid
- 2,6-Dioxo-3-(5-O-phosphono-beta-delta-ribofuranosyl)-1,2,3,6-tetrahydropyrimidine-4-carboxylate
- 2,6-Dioxo-3-(5-O-phosphono-beta-delta-ribofuranosyl)-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid
- 2,6-dioxo-3-(5-O-phosphono-β-D-Ribofuranosyl)-1,2,3,6-tetrahydropyrimidine-4-carboxylate
- 2,6-dioxo-3-(5-O-phosphono-β-D-Ribofuranosyl)-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid
- 2,6-dioxo-3-(5-O-phosphono-β-δ-Ribofuranosyl)-1,2,3,6-tetrahydropyrimidine-4-carboxylate
- 2,6-dioxo-3-(5-O-phosphono-β-δ-Ribofuranosyl)-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid
- 5'-(Dihydrogen phosphate) 6-carboxy-uridine
- 5'-(Dihydrogen phosphate) Orotidine
- 5'-(Dihydrogen phosphoric acid) 6-carboxy-uridine
- 5'-(Dihydrogen phosphoric acid) orotidine
- 5'-OMP
- 5'-Phosphate Orotidine
- 5'-Phosphoric acid orotidine
- 5-(Dihydrogen phosphate)orotidine
- 5-(Dihydrogen phosphoric acid)orotidine
- 6-Carboxy-5'-uridylate
- 6-Carboxy-5'-uridylic acid
- Ometoprim
- OMP
- Omp (nucleotide)
- Orotidine 5'-(dihydrogen phosphate)
- Orotidine 5'-(dihydrogen phosphoric acid)
- Orotidine 5'-monophosphate
- Orotidine 5'-monophosphoric acid
- Orotidine 5'-phosphate
- Orotidine 5'-phosphoric acid
- Orotidine monophosphate
- Orotidine monophosphoric acid
- Orotidine-5'-phosphate
- Orotidine-5'-phosphoric acid
- Orotidine5'P
- Orotidylate
- Orotidylic acid
|
|---|
|
Chemical Formula: |
C10H13N2O11P |
|---|
| Average Molecular Weight: |
368.1908 |
|---|
| Monoisotopic Molecular
Weight: |
368.02569578 |
|---|
| InChI Key: |
KYOBSHFOBAOFBF-XVFCMESISA-N |
|---|
| InChI: | InChI=1S/C10H13N2O11P/c13-5-1-3(9(16)17)12(10(18)11-5)8-7(15)6(14)4(23-8)2-22-24(19,20)21/h1,4,6-8,14-15H,2H2,(H,16,17)(H,11,13,18)(H2,19,20,21)/t4-,6-,7-,8-/m1/s1 |
|---|
| CAS
number: |
2149-82-8 |
|---|
| IUPAC Name: | 3-[(2R,3R,4S,5R)-3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]-2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid |
|---|
|
Traditional IUPAC Name: |
6-carboxy-5'-uridylic acid |
|---|
| SMILES: | O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP(O)(O)=O)N1C(=O)NC(=O)C=C1C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pyrimidine ribonucleoside monophosphates. These are pyrimidine ribobucleotides with monophosphate group linked to the ribose moiety. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
|
Class |
Pyrimidine nucleotides |
|---|
| Sub Class | Pyrimidine ribonucleotides |
|---|
|
Direct Parent |
Pyrimidine ribonucleoside monophosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidine ribonucleoside monophosphate
- N-glycosyl compound
- Glycosyl compound
- Monosaccharide phosphate
- Pyrimidine-6-carboxylic acid or derivatives
- Pyrimidine-6-carboxylic acid
- Hydropyrimidine carboxylic acid derivative
- Hydroxypyrimidine
- Monoalkyl phosphate
- Pyrimidone
- Alkyl phosphate
- Pyrimidine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- Monosaccharide
- Hydropyrimidine
- Heteroaromatic compound
- Oxolane
- Secondary alcohol
- 1,2-diol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
- pyrimidine ribonucleoside 5'-monophosphate (CHEBI:15842 )
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. Pubmed: 19212411
- Traut TW: Uridine-5'-phosphate synthase: evidence for substrate cycling involving this bifunctional protein. Arch Biochem Biophys. 1989 Jan;268(1):108-15. Pubmed: 2912371
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: |
Ueda, Tohru; Yamamoto, Miyako; Yamane, Akira; Imazawa, Masaoki; Inoue, Hideo. Nucleosides and nucleotides. XXIII. Conversion of uridine nucleotides to the 6-cyano derivatives: synthesis of orotidylic acid. Journal of Carbohydrates, Nucleosides, Nuc |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|