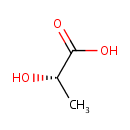
L-Lactic acid (PAMDB000079)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000079 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | L-Lactic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Lactic acid plays a role in several biochemical processes. Pseudomonas aeruginosa produces lactic acid from pyruvate in a process of normal metabolism. The lactic acid produced by Pseudomonas aeruginosa and other probiotics (mostly lactic acid bacteria) can lower the pH in mammalian host intestines and therefore, it inhibits the growth of proteolytic bacteria and have a beneficial effect on intestinal inflammation. (PMID 15138208) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C3H6O3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 90.0779 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 90.031694058 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | JVTAAEKCZFNVCJ-REOHCLBHSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C3H6O3/c1-2(4)3(5)6/h2,4H,1H3,(H,5,6)/t2-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 79-33-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2S)-2-hydroxypropanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | (α)-lactate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | C[C@H](O)C(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as alpha hydroxy acids and derivatives. These are organic compounds containing a carboxylic acid substituted with a hydroxyl group on the adjacent carbon. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Hydroxy acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Alpha hydroxy acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Alpha hydroxy acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Liquid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 16.8 °C (BP = 119 oC) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | L-Lactic acid + Ubiquinone-8 > Pyruvic acid + Ubiquinol-8 L-Lactic acid + Menaquinone 8 > Menaquinol 8 + Pyruvic acid Water + Lactaldehyde + NAD + (S)-Lactaldehyde <>2 Hydrogen ion + L-Lactic acid + NADH L-Lactic acid + 2 Ferricytochrome c + Ferricytochrome c <> Pyruvic acid +2 Ferrocytochrome c +2 Hydrogen ion + Ferrocytochrome c Lactaldehyde + NAD + Water <> L-Lactic acid + NADH + Hydrogen ion an oxidized electron acceptor + L-Lactic acid > a reduced electron acceptor + Pyruvic acid Pyruvic acid + Hydrogen ion > L-Lactic acid Lactaldehyde + NAD + Water > L-Lactic acid + NADH L-Lactic acid + 2 Ferricytochrome c > Pyruvic acid +2 Ferrocytochrome c +2 Hydrogen ion NAD + Water + (S)-lactaldehyde + Lactaldehyde > NADH +2 Hydrogen ion + L-Lactic acid + L-Lactic acid L-Lactic acid + oxidized electron acceptor + L-Lactic acid > Reduced acceptor + Pyruvic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Lao, Hanzhang; Sun, Jianrong; Wang, Jian; Qian, Zhiliang. Process for preparation of high-purity L-lactic acid. Faming Zhuanli Shenqing Gongkai Shuomingshu (2007), 9pp. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in catalytic activity
- Specific function:
- (S)-lactate + 2 ferricytochrome c = pyruvate + 2 ferrocytochrome c + 2 H(+)
- Gene Name:
- lldD
- Locus Tag:
- PA4771
- Molecular weight:
- 41.1 kDa
Reactions
| (S)-lactate + 2 ferricytochrome c = pyruvate + 2 ferrocytochrome c + 2 H(+). |
Transporters
- General function:
- Involved in lactate transmembrane transporter activity
- Specific function:
- Transports L-lactate across the membrane. Can also transport D-lactate and glycolate. Seems to be driven by a proton motive force
- Gene Name:
- lldP
- Locus Tag:
- PA4770
- Molecular weight:
- 58.7 kDa