|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000004 |
|---|
|
Identification |
|---|
| Name: |
a-Ketoisovaleric acid |
|---|
| Description: | a-Ketoisovaleric acid is a precursor of valine, leucine and pantothenate. It can be synthesized in Pseudomonas aeruginosa. anabolically (via dihydroxyacid dehydrase) and catabolically (branched-chain amino acid transaminase and alanine-valine transaminase) |
|---|
|
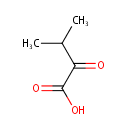
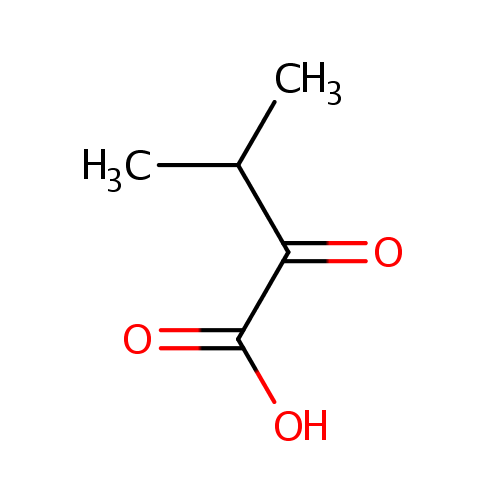
Structure |
|
|---|
| Synonyms: | - 2-Keto-3-Methylbutyrate
- 2-Keto-3-Methylbutyric acid
- 2-Ketoisovalerate
- 2-Ketoisovaleric acid
- 2-Oxo-3-methylbutanoate
- 2-Oxo-3-methylbutanoic acid
- 2-Oxo-3-methylbutyrate
- 2-Oxo-3-methylbutyric acid
- 2-Oxoisovalerate
- 2-Oxoisovaleric acid
- 3-Methyl-2-oxo-Butanoate
- 3-Methyl-2-oxo-Butanoic acid
- 3-Methyl-2-oxo-Butyrate
- 3-Methyl-2-oxo-Butyric acid
- 3-Methyl-2-oxobutanoate
- 3-Methyl-2-oxobutanoic acid
- 3-Methyl-2-oxobutyrate
- 3-Methyl-2-oxobutyric acid
- A-Keto-b-Methylbutyrate
- a-Keto-b-Methylbutyric acid
- A-Keto-Isovalerate
- a-Keto-Isovaleric acid
- A-Ketoisovalerate
- A-Oxo-b-methylbutyrate
- a-Oxo-b-methylbutyric acid
- A-Oxoisovalerate
- a-Oxoisovaleric acid
- alpha-Ketoisovalerate
- alpha-Ketoisovaleric acid
- Dimethylpyruvate
- Dimethylpyruvic acid
- Isopropylglyoxylate
- Isopropylglyoxylic acid
- Ketovaline
- α-Ketoisovalerate
- α-Ketoisovaleric acid
|
|---|
|
Chemical Formula: |
C5H8O3 |
|---|
| Average Molecular Weight: |
116.1152 |
|---|
| Monoisotopic Molecular
Weight: |
116.047344122 |
|---|
| InChI Key: |
QHKABHOOEWYVLI-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C5H8O3/c1-3(2)4(6)5(7)8/h3H,1-2H3,(H,7,8) |
|---|
| CAS
number: |
759-05-7 |
|---|
| IUPAC Name: | 3-methyl-2-oxobutanoic acid |
|---|
|
Traditional IUPAC Name: |
α-ketoisovalerate |
|---|
| SMILES: | CC(C)C(=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as short-chain keto acids and derivatives. These are keto acids with an alkyl chain the contains less than 6 carbon atoms. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Keto acids and derivatives |
|---|
| Sub Class | Short-chain keto acids and derivatives |
|---|
|
Direct Parent |
Short-chain keto acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Methyl-branched fatty acid
- Short-chain keto acid
- Branched fatty acid
- Fatty acyl
- Alpha-keto acid
- Alpha-hydroxy ketone
- Ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
31.5 °C |
|---|
| Experimental Properties: |
| Property | Value | Source |
|---|
| Water Solubility: | 0.1 g/mL MeOH hot clear | PhysProp | | LogP: | 0.329 | PhysProp |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Alanine, aspartate and glutamate metabolism pae00250
- Metabolic pathways pae01100
- Pantothenate and CoA biosynthesis pae00770
- Pyruvate metabolism pae00620
- Valine, leucine and isoleucine biosynthesis pae00290
- Valine, leucine and isoleucine degradation pae00280
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-000i-9500000000-ff936b879a69b5d118f8 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-000i-8920000000-e37b37d64d43dcf763f0 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 1 TMS) | splash10-000i-9400000000-e3995acc4818a98d0f48 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 1 TMS) | splash10-0f79-9720000000-5d89487273e44ea61a68 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000i-9000000000-10ab58a33e9ca7dbace0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-000i-9000000000-ad51ff01c94b6046ad64 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-014i-0900000000-9993174a7b1801b90ddb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-00xr-9500000000-293818b81e0879b6feb2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-00di-9000000000-75058f27a2178b9cf121 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-0006-9000000000-4c20af39e8ee009d5278 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00r2-9400000000-21f8c3fae79161c82099 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-9000000000-5f25e41413738bb6b5d7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-801af00dea93fcfd637d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01b9-8900000000-5185c7dfc72c25069904 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00xs-9200000000-7e2a275a65197f96f5d7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0600-9000000000-0013e0ff06f9896a1337 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Chuang DT, Niu WL, Cox RP: Activities of branched-chain 2-oxo acid dehydrogenase and its components in skin fibroblasts from normal and classical-maple-syrup-urine-disease subjects. Biochem J. 1981 Oct 15;200(1):59-67. Pubmed: 6895847
- Gallina DL, Dominguez JM, Hoschoian JC, Barrio JR: Maintenance of nitrogen balance in a young woman by substitution of -ketoisovaleric acid for valine. J Nutr. 1971 Sep;101(9):1165-7. Pubmed: 5096137
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Lee SH, Kim SO, Chung BC: Gas chromatographic-mass spectrometric determination of urinary oxoacids using O-(2,3,4,5,6-pentafluorobenzyl)oxime-trimethylsilyl ester derivatization and cation-exchange chromatography. J Chromatogr B Biomed Sci Appl. 1998 Nov 20;719(1-2):1-7. Pubmed: 9869358
- Livesey G, Lund P: Binding of branched-chain 2-oxo acids to bovine serum albumin. Biochem J. 1982 Apr 15;204(1):265-72. Pubmed: 7115325
- Schaefer K, von Herrath D, Erley CM, Asmus G: Calcium ketovaline as new therapy for uremic hyperphosphatemia. Miner Electrolyte Metab. 1990;16(6):362-4. Pubmed: 2089249
- Schauder P, Schroder K, Langenbeck U: Serum branched-chain amino and keto acid response to a protein-rich meal in man. Ann Nutr Metab. 1984;28(6):350-6. Pubmed: 6393856
- Shigematsu Y, Kikuchi K, Momoi T, Sudo M, Kikawa Y, Nosaka K, Kuriyama M, Haruki S, Sanada K, Hamano N, et al.: Organic acids and branched-chain amino acids in body fluids before and after multiple exchange transfusions in maple syrup urine disease. J Inherit Metab Dis. 1983;6(4):183-9. Pubmed: 6422161
- Shoemaker JD, Elliott WH: Automated screening of urine samples for carbohydrates, organic and amino acids after treatment with urease. J Chromatogr. 1991 Jan 2;562(1-2):125-38. Pubmed: 2026685
- Tsuchiya H, Hashizume I, Tokunaga T, Tatsumi M, Takagi N, Hayashi T: High-performance liquid chromatography of alpha-keto acids in human saliva. Arch Oral Biol. 1983;28(11):989-92. Pubmed: 6581765
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
- Yurtsever D. (2007). Fatty acid methyl ester profiling of Enterococcus and Esherichia coli for microbial source tracking. M.sc. Thesis. Villanova University: U.S.A
|
|---|
| Synthesis Reference: |
Bouveault, L. etal., Bull. Soc. Chim. Fr., 1901, 1031; Singh, J. etal., Org. Prep. Proced. Int., 1989, 21, 501; Hata, H. etal., Synthesis, 1991, 289 |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | 16530 | | HMDB ID | HMDB00019 | | Pubchem Compound ID | 49 | | Kegg ID | C00141 | | ChemSpider ID | 48 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available | | Ligand Expo | KIV |
|
|---|