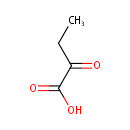
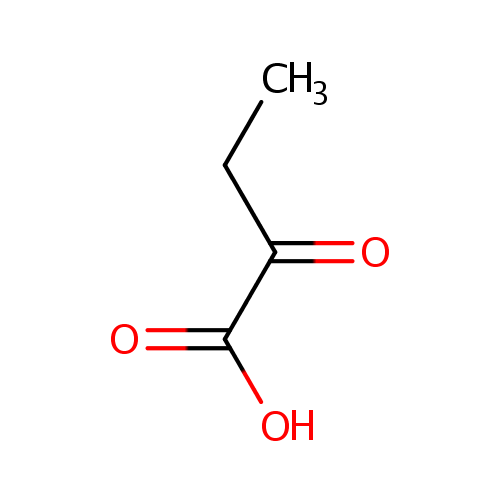
2-Ketobutyric acid (PAMDB000001)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | 2-Ketobutyric acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | 2-Ketobutyric acid (alpha ketobutyric acid) is involved in the metabolism of many amino acids (glycine, cysteine, methionine, valine, leucine, serine, threonine, isoleucine). It also plays a role in propanoate metabolism and C-5 branched dibasic acid metabolism. More specifically, alpha-ketobutyric acid can be produced through the lysis of cystathionine (via cystathionine gamma lyase) leading to the production of cysteine and alpha-ketobutyric acid. It is also one of the degradation products of threonine. It can be converted to propionyl-CoA (and subsequently methylmalonyl CoA, which can be converted to succinyl CoA, a citric acid cycle intermediate), and thus enter the citric acid cycle. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C4H6O3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 102.0886 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 102.031694058 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | TYEYBOSBBBHJIV-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C4H6O3/c1-2-3(5)4(6)7/h2H2,1H3,(H,6,7) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 600-18-0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 2-oxobutanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | 2-oxobutanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CCC(=O)C(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as short-chain keto acids and derivatives. These are keto acids with an alkyl chain the contains less than 6 carbon atoms. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Keto acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Short-chain keto acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Short-chain keto acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 33°C [Suante, H.; Oxidation Communications 2004, V27(2), P344-348] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 2-Ketobutyric acid + Coenzyme A > Formic acid + Propionyl-CoA 2-Ketobutyric acid + Hydrogen ion + Pyruvic acid > 2-Aceto-2-hydroxy-butyrate + Carbon dioxide L-Threonine > 2-Ketobutyric acid + Ammonium 2-Ketobutyric acid + Carbon dioxide + NADH + Hydrogen ion <> D-Erythro-3-Methylmalate + NAD L-Threonine <> 2-Ketobutyric acid + Ammonia O-Succinyl-L-homoserine + Water <> 2-Ketobutyric acid + Succinic acid + Ammonia Pyruvic acid + 2-Ketobutyric acid <> 2-Aceto-2-hydroxy-butyrate + Carbon dioxide Hydrogen ion + 2-Ketobutyric acid + Succinic acid + Ammonia O-Succinyl-L-homoserine + Water an aminated amine donor + 2-Ketobutyric acid + Hydrogen ion 2-aminobutyrate + a deaminated amine donor L-Threonine > Hydrogen ion + 2-Ketobutyric acid + Ammonia Iminobutyrate + Water > 2-Ketobutyric acid + Ammonia L-Threonine + 2-Aminobut-2-enoate + 2-Iminobutanoate + Water <> 2-Ketobutyric acid + Ammonia 2-Ketobutyric acid + Coenzyme A > Formic acid + Propionyl-CoA + Propionyl-CoA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Figge, Rainer; Lux, Fabien; Raynaud, Celine; Soucaille, Philippe. Production of a-ketobutyrate by engineered Escherichia coli.PCT Int. Appl. (2006), 31pp. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||